Element vs Compound
Element vs Compound
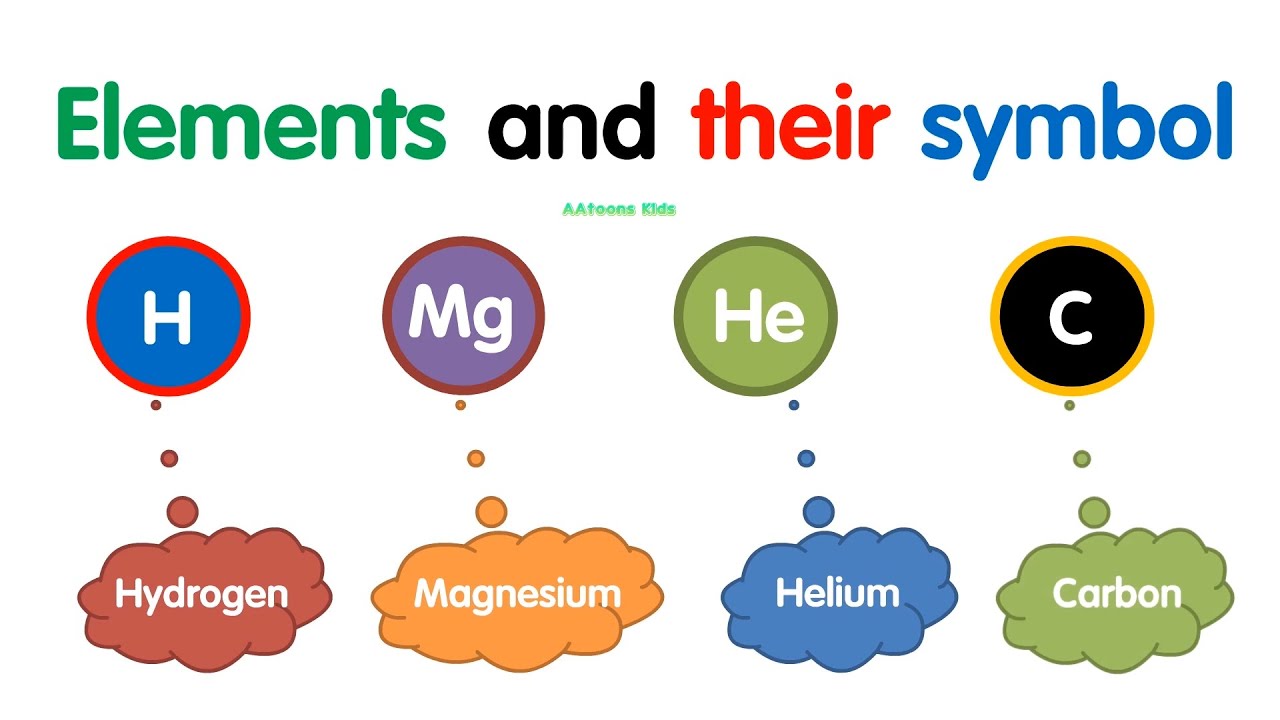
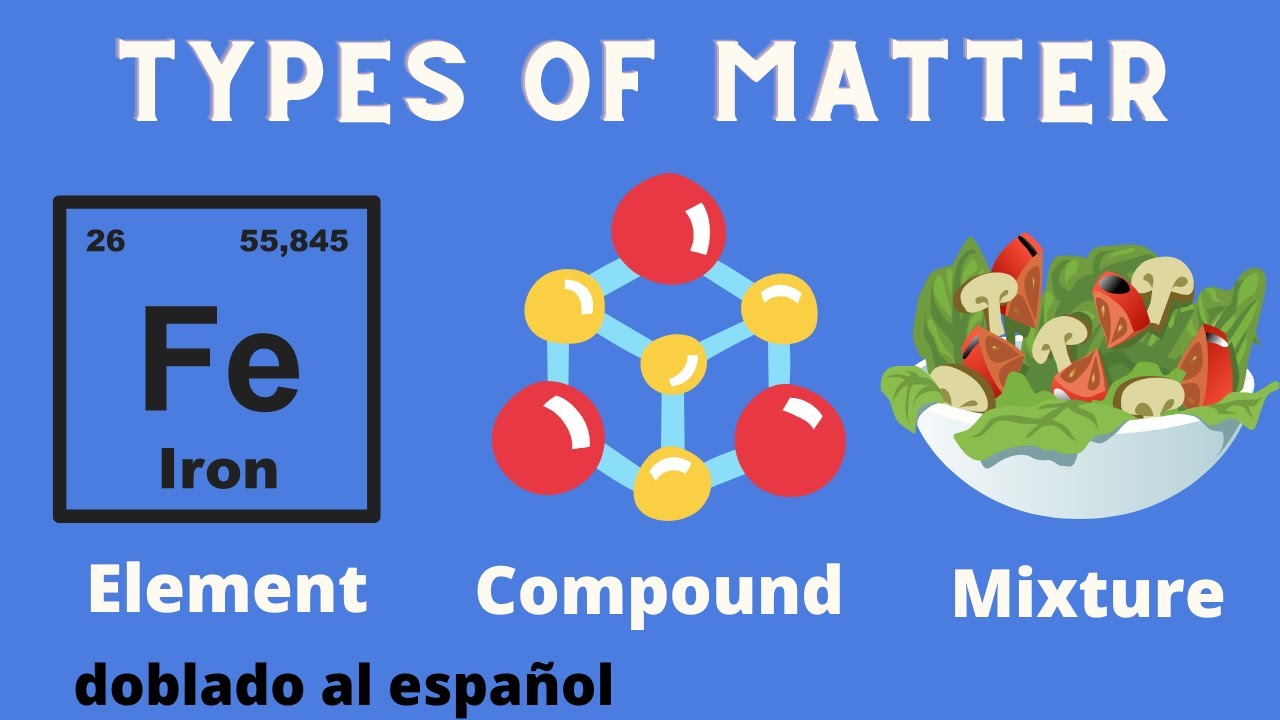
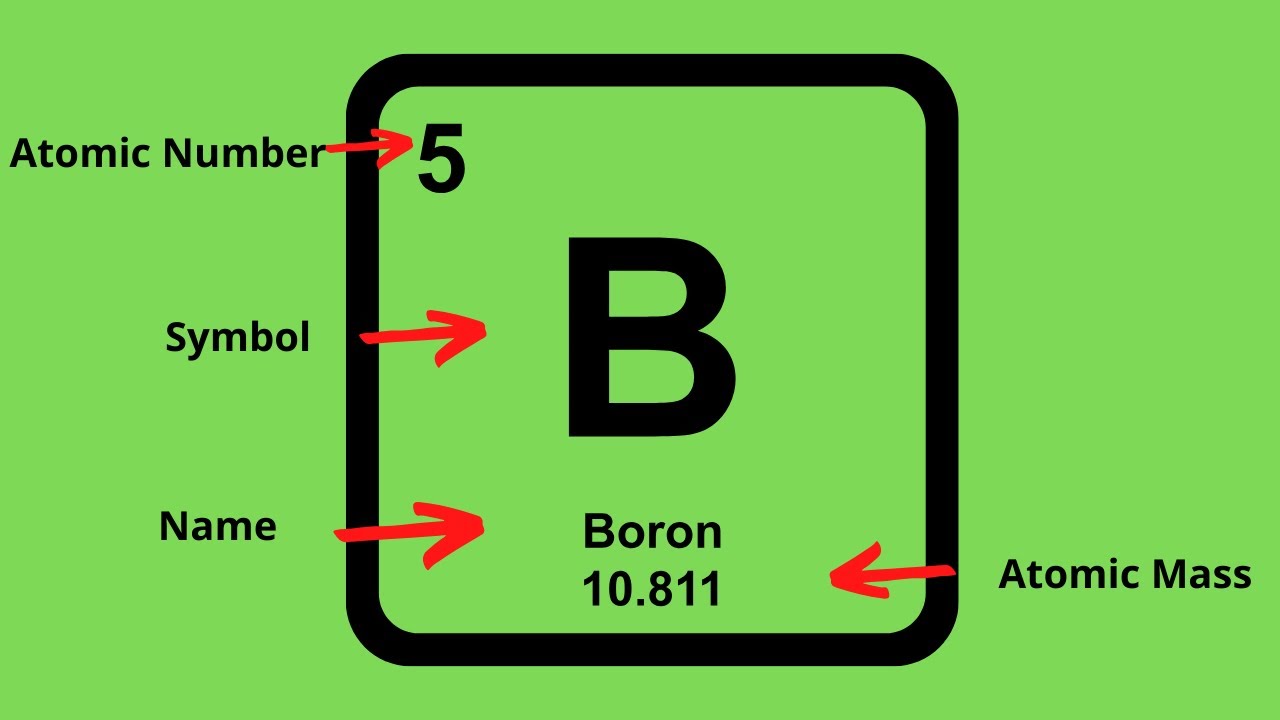
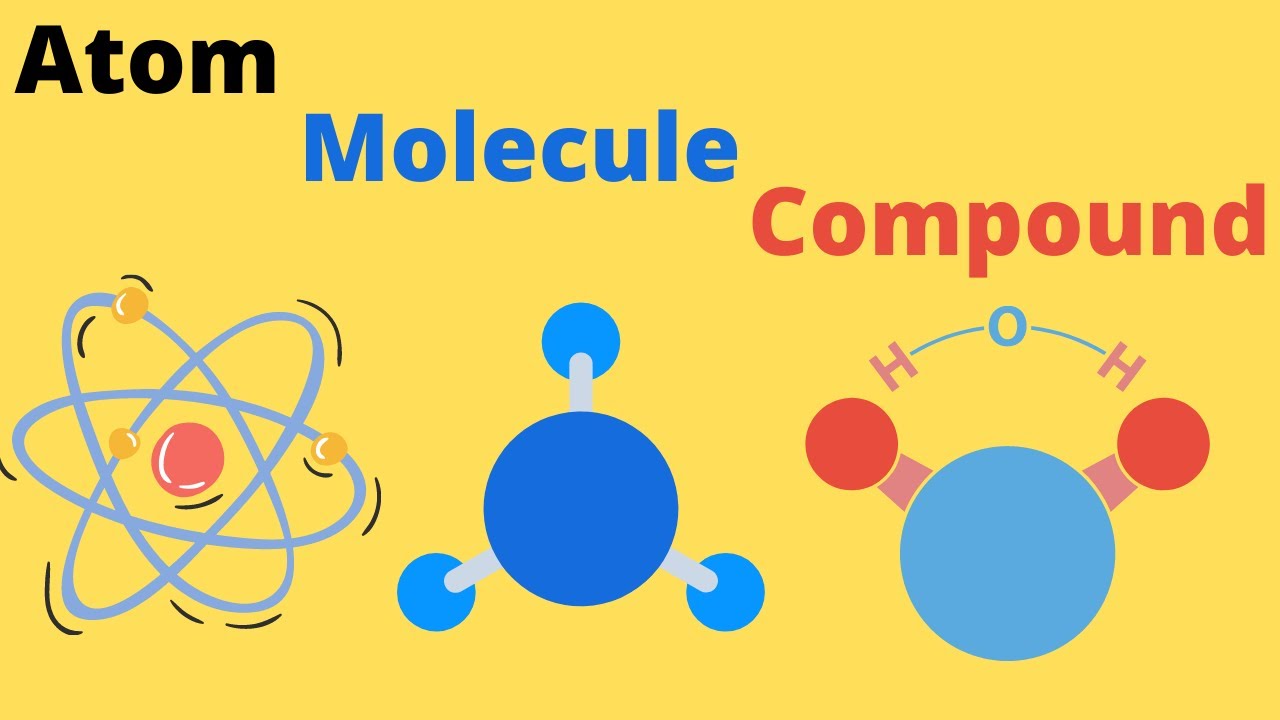
An element is a pure substance. In other words, an element cannot be broken down into a simpler substance by physical or chemical means.An element is made up of only one type of atom. An atom consists of a proton, neutron, and an electron. Some common examples of an element would be carbon, oxygen, or iron. The elements are arranged in the periodic table based on their number of protons. For example, sodium has 11 protons, potassium has 19
The number of protons of elements always stays the same.
Each element also has its own unique set of properties.
A compound is a pure substance of two or more elements chemically combined. Elements combine during a chemical change. A particle of a compound is called a molecule.
When the elements combine chemically a new substance is created.
The compound has different properties than the elements that make it
Hydrogen and oxygen are gases but they combine to make liquid water.
Here are some items that combine to make compounds
Table salt is sodium and chlorine
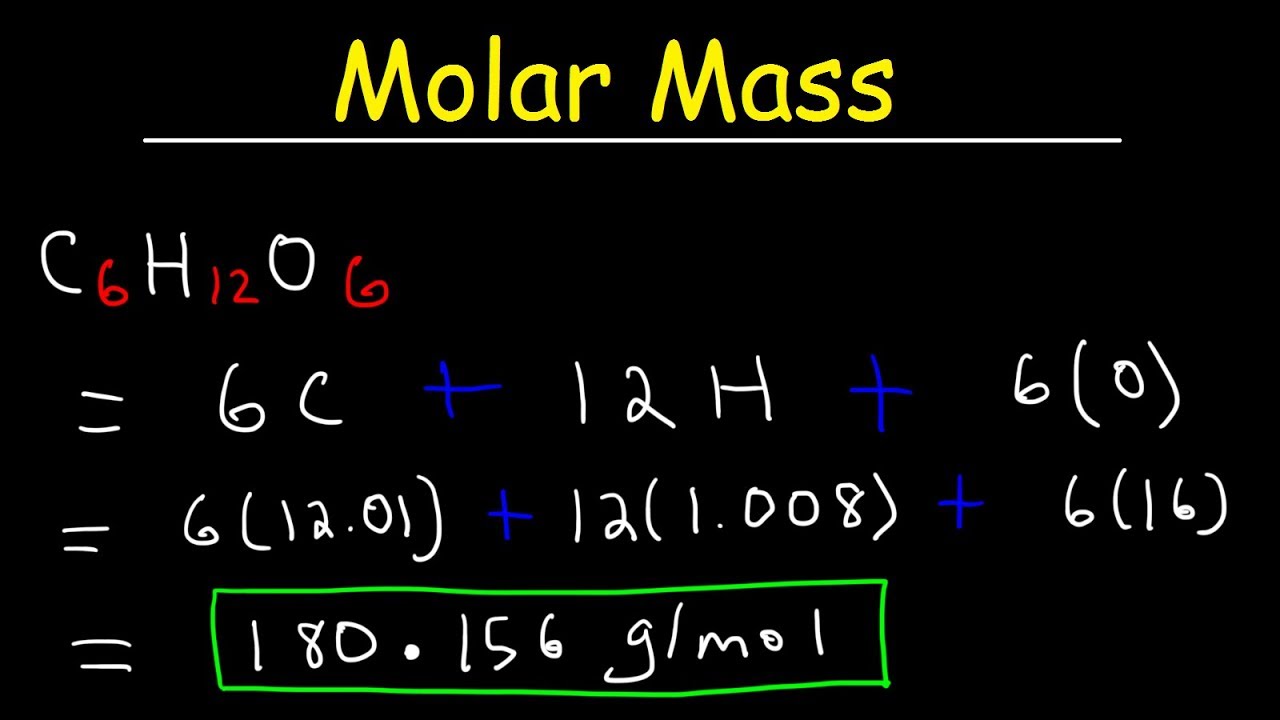
Glucose is made of carbon-hydrogen-oxygen
When elements create a compound they always join in a specific ratio
The ratio of water is hydrogen to oxygen is 2 to 1
If a compound has a different ratio of hydrogen to oxygen then it is not water
Glucose a type of sugar has a ratio of c6 h12 o6 which is 6 carbons 12 hydrogens and 6 oxygens
Glucose will always have this same ratio
Compounds are all around you
In the food you eat, the school supplies you use, your clothes and even you