סרטונים מובילים
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Welcome our new video: Learn How to Read | How to Teach Your Child to Read | Kids Academy
Subscribe to our channel: https://goo.gl/iG2Bdr
Check our new videos of learning how to read is funny and interactive.
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
Subscribe to our channel: https://goo.gl/iG2Bdr
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Learn How to Read | How to Teach Your Child to Read
Teaching a kid to read can be a challenge sometimes. It is mostly because parents take a lot more pressure of reading of the kids. Because the kids will eventually learn to speak and recognize the colors and other things around them, but reading is a major milestone.
While some kids learn to read very quickly, some kids might take some time to adapt to the new thing that they are learning. It is very important that the kid is given enough time and attention to grasp to the new ideas. Putting a lot of pressure on them will only make them dislike this whole thing and they might not even put that much effort. It is very important that the parents and the teachers understand the nature of each individual child because every child is different and needs a different kind of attention.
With that being said, the next step is how to teach your child to read. It is very important that this new learning starts at home. Because is the child’s first school and the parents their first teachers. So, set a good example.
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Telling Time Word Problems | Math | 1st Grade
When it comes to math problems, telling the time in word problems is different from other math problems. That’s because minutes and hours are counted in units of 60, making counting time tricky to figure out. Give your child valuable practice using this helpful video that teaches kids how to add to count time.
While watching, your child will observe:
- Our teacher adding the time using a familiar addition method
- Frequent reminders of how to count units of time
Telling time in word problems doesn’t have to be more difficult than any other word problem. This video will assist your child in remembering how to count time, while offering a familiar strategy to solve the problems!
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
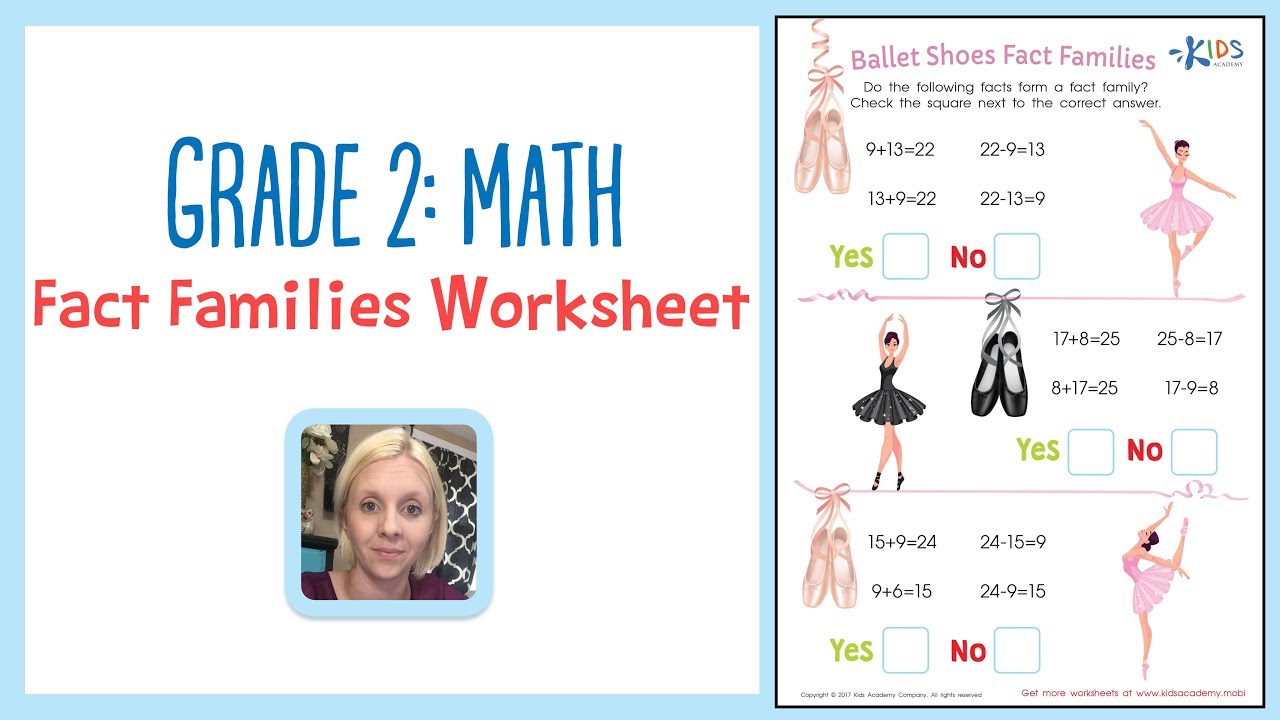
Fact families are fun to learn, but most importantly, they help kids learn to analyze numbers in relation to one another in grade 2 math! Your child will dance through this ballet-themed fact families worksheet as they watch this instructional video tutorial to learn reinforce addition and subtraction skills.
As your child watches, he or she will learn to:
• Draw a picture of house to use as a visual aid to analyze fact families
• Analyze number facts in a variety ways, including addition and subtraction
• Relate addition to subtraction through the use of fact families
Fact families aren’t just fun, but they help kids grow their number sense as they learn to analyze math facts!
Grade 2: Math—Add Ones and Tens
Grade 2 math focuses on helping kids understand math facts as they learn to add and subtract. Using this engaging video, your child will add ones and tens as they balance equations with the guidance of an experienced elementary teacher!
While watching this video, your child will:
• Recall prior knowledge of fact families
• Observe a visual strategy using dots to solve simple problems
• Learn to relate subtraction with addition to balance the equations
Sit back and watch as your child learns to balance equations in no time! With the help of a trusted teacher, your child will learn the right techniques to solve each problem!
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
It's Kids Academy, and welcome back to our channel! When school is back, your kid’s ahead! Get your kids excited about going back to school with Kids Academy Back-to-School Program! Try Kids Academy with 3-day FREE trial - reserve your spot now! https://bit.ly/31f2fGy
See the collection of nursery songs with Wheels on the Bus, Baa Baa Black Sheep, Down by the Bay, Finger Family and London Bridge by Kids Academy! Kids and adults are never tired or bored by these long-lasting nursery rhyme songs which serve to benefit your kids’ educational all-rounded development as well as bring entertainment and fun into the classroom.
Get the most from learning with our new nursery rhyme playlist for children and start getting joy and pleasure right now!
Begin or finish your lesson with Wheels on the Bus and your toddlers will be definitely in the mood to study and learn more. Not only the rhyme is loved by kids thanks to the catchy tune and funny plot but it also improves kids’ word comprehension, reading and writing skills.
Finger Family is one of the most popular nursery rhyme songs with lyrics and action that preschoolers love to sing and do over and over again. It’s a lovely action and finger play nursery rhyme about fingers with a family on it. Kids will be excited to sing along to the song learning the body parts, colors, shapes and the names of the fingers. In search of activities combining word recognition and pronunciation, practice do not overlook the nursery rhyme about Baa Baa Black Sheep. Use it to revise the sounds various animals produce or practice reading where kids learn to recognize and read the lyrics to Baa Baa Black Sheep.
Down by the Bay is perfect for developing basic literacy and communication skills in your little ones. The song has a great choice of rhyming words which will be ideal for various purposes: recognizing between multiple sounds, building and improving vocabulary, getting more practice on rhythm and intonation, and basic body actions (clapping, jumping, tapping, patting your legs, tummy or head, etc.). Its catchy tune is so adorable that your kids can listen to it on repeat for many times!
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Kids Academy presents a new animated version of the traditional kid’s Bingo Song. Let your kids watch the video and see how amazed and excited your little learners are! The song brings fun into every classroom and is enjoyed by kids of different ages. There’s a bunch of other captivating Bingo Song activities on our website!
👍Share this video and smash like button!👍
Lyrics:
There was a farmer who had a dog,
And Bingo was his name-o.
B-I-N-G-O
B-I-N-G-O
B-I-N-G-O
And Bingo was his name-o.
There was a farmer who had a dog,
And Bingo was his name-o.
(clap)-I-N-G-O
(clap)-I-N-G-O
(clap)-I-N-G-O
And Bingo was his name-o.
There was a farmer who had a dog,
And Bingo was his name-o.
(clap)-(clap)-N-G-O
(clap)-(clap)-N-G-O
(clap)-(clap)-N-G-O
And Bingo was his name-o.
There was a farmer who had a dog,
And Bingo was his name-o.
(clap)-(clap)-(clap)-G-O
(clap)-(clap)-(clap)-G-O
(clap)-(clap)-(clap)-G-O
And Bingo was his name-o.
There was a farmer who had a dog,
And Bingo was his name-o.
(clap)-(clap)-(clap)-(clap)-O
(clap)-(clap)-(clap)-(clap)-O
(clap)-(clap)-(clap)-(clap)-O
And Bingo was his name-o.
There was a farmer who had a dog,
And Bingo was his name-o.
(clap)-(clap)-(clap)-(clap)-(clap)
(clap)-(clap)-(clap)-(clap)-(clap)
(clap)-(clap)-(clap)-(clap)-(clap)
And Bingo was his name-o.
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Measurement for Kids - Capacity: Cups, Pints and Quarts | Kids Academy
Cups, pints, and quarts: measurement for kids sure is tricky when there are so many ways to measure liquids! This informative video tutorial will clear up any confusion as your child watches a real teacher talk through each problem! In addition to the chart on the worksheet, our teacher offers even more visuals to reinforce learning and memorization.
Watching this video will benefit your child because:
• Our teacher will guide your child through each problem and answer choice
• Thoroughly explain each answer choice using pictures to prove answers
Learning measurement depends on visual aids to make it understandable to kids. Maximize your child’s learning and increase his or her skills with video tutorials like this one from Kids Academy!
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Welcome to the Kids Academy channel! Today we will talk about the 4th of July for kids. Independence Day is celebrated on the anniversary of national independence. It is the day when a nation was declared a free nation and got its right to make laws of its state.
Many countries in this world were once a part of some other monarchy or a bigger power. But after fighting for independence they got their own national identity and emerged on the world map as a separate independent nation.
It is important for kids to learn what is Independence Day and the Independence Day history of their own country. Every country has its own story of how they got their independence and becomes a part of the nation’s history.
For example, America got its independence from Great Britain on the 4th of July 1776. Since then the day is celebrated as the anniversary of America’s independence.
Similarly, the partition of subcontinent gave birth to two independent countries, India and Pakistan who got their independence from the British.
There are many other examples of Independence Day, every kid must know about their own country’s independence history.
Subscribe to our channel: https://goo.gl/iG2Bdr
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Learn The English Alphabet for Kids | Kids Academy
Install now Kids Academy Talented & Gifted: https://smart.link/5d9b5460c7805
Getting familiar with all the alphabets in the English language is very important for your children as it paves a proper way for them on which they can lay the whole foundation of their initial learning. That is why today we will learn the alphabets starting from A all the way to H.
A
A is the first alphabet in the English language and the kids can develop an understanding for it through leaning various words that start by it:
• Apple
• Avocado
• Animal
B
The 2nd alphabet in the English language and often a favorite of children as it is not so hard to grasp, understanding a few words that start from it can certainly help;
• Ball
• Banana
• Bag
C
It is the 3rd alphabet in the English literature and in course of providing alphabet for kids, kids academy focuses over providing them with the words that start by it.
• Cat
• Cauliflower
• Carrot
D
In the English Alphabet for kids, this is the 4th alphabet.
• Drum
• Duck
• Door
E
It is the 5th alphabet in the English literature, the following words might prove beneficial for kids to learn from;
• Eagle
• Egg
• Eggplant
F
The 5th letter in English alphabets is the word F;
• Fish
• Finger
• Flower
G
It is the 6th alphabet in the English alphabets and contains the maximum amount of words;
• Goat
• Grapes
• Globe
H
The 7th alphabet in the English language;
• Hen
• Helicopter
• Hand
Subscribe to our channel: https://goo.gl/iG2Bdr
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyC...
Twitter: https://twitter.com/KidsAcademyCo
Hello, this is a new video from Kids Academy and we decided to make a quiz for kids about vegetables and interesting facts about them. If you want to learn new facts about vegetables, you should watch this interesting video for kids.
Subscribe to our channel: https://goo.gl/iG2Bdr
#kidsacademy #LearnWithKidsAcademy #vegetables
Connect with us on :
App Store: https://apps.apple.com/app/apple-store/id543851593
Google Play: https://play.google.com/store/....apps/details?id=air.
Our website: http://www.kidsacademy.mobi
Instagram: https://www.instagram.com/kidsacademyco
Facebook: https://www.facebook.com/KidsAcademyCompany
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Sorting Games, Matching Games, Logic Games for Kids
Install now Kids Academy Talented & Gifted: https://smart.link/5d9b5460c7805
Here at Kid’s Academy, we know that improving young students’ critical thinking skills helps them become stronger students when they enter primary school. These videos teach students observational skills, reasoning skills and logical thinking with sorting games and matching games. And very young children begin learning first with color games.
Games and instruction for toddlers and students in preschool and Kindergarten begin with learning about colors. Color games for kids include not only naming colors but also matching objects of the same color. Once students master color, they begin to work on sorting games and look at the size and then shape to describe, sort and classify objects. Language skills improve as well because students have the words to describe the world around them.
As students progress, students use higher-order thinking skills to determine if there is more than one way to sort objects and use reasoning skills to decide if objects go together and why they go together.
Students enjoy learning with the child-friendly instruction on these clips. Skills and concepts include:
• Matching colors.
• identifying toys that are the “same” and “different.”
• Objects that fit together. A fish and water go together, and cookies and milk fit together. Students use logic and reasoning to find other things that go together.
• Finding the object that is different in a group of objects. Students look for differences in size, shape, color or position.
• Matching objects that are exactly the same.
• Sorting objects by size.
• Sorting objects in a group two different ways—by size and color.
Kid’s Academy’s logic games for kids are fun! Children want to watch and play more and more! The videos are a valuable tool for the classroom as well as for parents wanting to help their children improve their thinking and reasoning skills.
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Facebook: https://www.facebook.com/KidsAcademyC...
Twitter: https://twitter.com/KidsAcademyCo
Install for free our Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/5a1bea3272481
Our website: https://www.kidsacademy.mobi/
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Learn to Play Chess | What is Pins? | Chess Tactics
What are the pins? The pin is amongst most common chess tactics. Pins happen when we are attacking a piece and a more valuable piece s revealed behind it.
Pins only happen in a straight line and for long-range pieces which means rooks, bishops and queens can make this move. Pawns, knights and kings can unfortunately not make this move.
It mostly happens when you attack a piece like a knight by a rook, but there is king behind this knight.
So, the knight is now pinned between a rook and the king. It cannot be moved because if the knight moves the king, which is the most valuable piece in chess, will be captured and it will be checkmate. So instantly you lose the game.
However, you need to play this move very carefully because you end up losing more points because when you capture through a pin you can put yourself in a threat by some other piece lying just there in position to capture your piece. So if you are teaching chess for kids beginners teach them to always be alert in chess.
Subscribe to our channel: https://goo.gl/iG2Bdr
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyC...
Twitter: https://twitter.com/KidsAcademyCo
Did you know that in one experiment the African Gray Parrot outperformed five-year-old children in cognitive tasks?
But they're not alone in the intelligence game. Join us as we unravel the extraordinary abilities of animals that will leave you in awe.
00:00 - Start
00:35 - Dolphins
01:05 - Elephants
01:29 - Octopus
02:08 - African Grey Parrots
02:34 - Border Collies
02:57 - Chimpanzees
03:28 - New Caledonian Crows
03:47 - Rats
Subscribe to our channel: https://goo.gl/iG2Bdr
#kidsacademy #LearnWithKidsAcademy
Connect with us on :
App Store: https://apps.apple.com/app/apple-store/id543851593
Google Play: https://play.google.com/store/....apps/details?id=air.
Our website: http://www.kidsacademy.mobi
Instagram: https://www.instagram.com/kidsacademyco
Facebook: https://www.facebook.com/KidsAcademyCompany
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Meet our chess lesson for kids: How King Moves and Captures | Chess Lessons | Kids Academy
King is the most important piece on the chess board, it is the leader of all the pieces and one who captures the king wins the game.
Now like all other pieces king can also move only in some certain ways. It can move only one step at a time. However, it can move in any direction as long as it is one step at a time. So, it can move diagonally, vertically, sideways, upwards and downwards, diagonally upwards and diagonally downwards.
The king also captures one step at a time. Which means that whatever piece is in the one step distance of the king, it will be captured by it. If there is a piece at a longer distance from the king, the king will have to move all the steps one at a time as long as it reaches that piece and then it captures it.
The king also needs to be protected by the other pieces on the board because if your king gets captured by the opponents’ king, then your game is over.
King is the most important piece on the chessboard and must be protected because with its capture the game ends.
So that was a quick chess lesson for beginners about king moves and how it captures.
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
It’s time to master the tricky sight words: there, those, these while watching an informative teacher-guided video from Kids Academy! Allow our teacher to guide your child through the recognition and usage of these tough lookalike words for meaningful reading and writing practice!
Welcome our new video: Sight Words: There, Those, These for 2nd Grade
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Valentine’s Day is a great time to show our loved ones how much we care about them. Cards, chocolates, and roses are the usual gifts on this day. But do you happen to know when and where the first Valentine’s Day celebration took place or who tends to receive the most cards? Do you have any idea how much Americans spend on chocolate gifts?
In this informational video by Kids Academy, you will find answers to all the questions above and will also learn more interesting facts about St. Valentine’s Day. Get ready to show big and small signs of affection to the people you love! Happy Valentine’s Day!
00:00 - Start
00:58 - 1. Valentine’s Day is the 2nd largest holiday for sending greeting cards
01:31 - 2. The most valentines are received by teachers
02:13 - 3. Millions of Valentine’s gifts are for pets
02:53 - 4. Americans spend over 1 billion dollars on chocolates for Valentine’s Day!
03:23 - 5. Millions of roses are grown each year just for Valentine’s Day.
03:54 - 6. The first Valentine’s Day chocolate box was created in the 19th century by Cadbury
04:33 - 7. Conversation heart candy was first created to be used as medicine
05:09 - 8. The heart shape as we know it wasn’t always used as a symbol for love
05:53 - 9. The first Valentine’s Day celebration took place in Paris in 1400.
06:39 - 10. Lovebirds aren’t just a figure of speech; they’re real birds!
Subscribe to our channel: https://goo.gl/iG2Bdr
#kidsacademy #LearnWithKidsAcademy #valentinesdayforkids
Connect with us on :
App Store: https://apps.apple.com/app/apple-store/id543851593
Google Play: https://play.google.com/store/....apps/details?id=air.
Our website: http://www.kidsacademy.mobi
Instagram: https://www.instagram.com/kidsacademyco
Facebook: https://www.facebook.com/KidsAcademyCompany
Welcome to Kids Academy!
In this video, we will continue to learn how to make fun and easy Easter craft for kids. We will create an adorable Easter basket using simple materials like paper, glue, and paint. The step-by-step instructions are easy to follow and perfect for children of all ages.
So, let's get crafting and make some bunnies-citing DIY Easter decorations!
This basket will be a great easter decoration. So happy Easter 2024 and wish you all the best!
Subscribe to our channel: https://goo.gl/iG2Bdr
#kidsacademy #LearnWithKidsAcademy
Connect with us on :
App Store: https://apps.apple.com/app/apple-store/id543851593
Google Play: https://play.google.com/store/....apps/details?id=air.
Our website: http://www.kidsacademy.mobi
Instagram: https://www.instagram.com/kidsacademyco
Facebook: https://www.facebook.com/KidsAcademyCompany
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Learn Numbers for Kids Teach Counting How To Learn Easy The Numbers For Kids
Install now Kids Academy Talented & Gifted: https://smart.link/5d9b5460c7805
If you are here right now, chances are that your little one is at that stage where he has to learn how to count. It seems like an easy task to teach a child counting numbers but the truth is that it’s not at all easy. Your child is too young at the moment and his brain can’t catch things as quick as you want to so a little patience is required here.
Don’t worry because every parent and every teacher has to go through this phase of teaching a child how to count numbers. How possibly difficult can it be if everyone is doing it, right? Well, if you do agree with us on this one then make sure to stay calm and patient right now and note down the things we are about to tell you.
In order to learn numbers for kids, you don’t have to get into any complications, in fact, all you need to do is to invest your time. Yes, you read that right! Your time is all your little one needs to learn to count for kids.
Most importantly, you can use online Youtube videos to teach your kid all about numbers, how to spell them and how to count them. The reason behind this is simply that online Youtube videos use visuals and audios that attract the children and they pick things up easily without getting bored.
Other than this, you can even opt for different color books with numbers on them and you can read different stories to your kids and play with them some games that help your child understand the concept of counting. Just be patient and we assure you that you will see some results soon.
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
English Grammar: Build a Sentence for 1st Grade - Kids Academy
Your child will ace grammar in English class with the help of this video tutorial that shows your child how to take scrambled words to build a sentence! Our teacher will help your child construct a sentence by thinking of the parts of speech and the order of each word.
Watching this video will benefit your child’s writing because:
Your child will study parts of speech and where they fall in a sentence
It sets the foundation for learning sentence structure in more depth
After kids learn how to build basic sentences, they can start studying the parts of a sentence, like subjects and objects, to write sentences with greater complexity. Use this video to get started studying and improving writing!
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
*****
Subscribe to our channel: https://goo.gl/iG2Bdr
Connect with us on :
Our site: https://www.kidsacademy.mobi/
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Now the kids can have fun learning questions and quotations marks with our teachers.
Welcome our new video: Congruent Shapes | 2nd Grade | Geometry | Kids Academy
#TalentedAndGifted #LearnWithKidsAcademy
Kids Academy Talented and Gifted Program for kids aged 2-10:
Subscribe to our channel: https://goo.gl/iG2Bdr
*****
Connect with us on :
App Store: https://smart.link/59833db06a6b8
Google Play: https://smart.link/597210af6eb83
Our website: http://www.kidsacademy.mobi
Facebook: https://www.facebook.com/KidsAcademyCompany
Twitter: https://twitter.com/KidsAcademyCo
Thousands of parents and educators are turning to the kids’ learning app that makes real learning truly fun. Try Kids Academy with
3-day FREE TRIAL! https://bit.ly/2GuGyL2
Kids learn fractions more easily when shown engaging pictures for a real-life connection! Your child will gobble up this video that features our expert teacher navigating through a tantalizing pizza-themed worksheet. Our teacher will help your child look at each piece of the pie to determine the correct answer!
Subscribe to our channel: https://goo.gl/iG2Bdr
Here’s how this video will help your child:
Our teacher will model how to match written fractions to the pizza pictures
The teacher’s think-aloud strategy will help your child understand fractions in more meaningful way
Use this video to help your child understand fractions better than ever before!