Science


After passing through the small intestine food travels into the large intestine.
Your large intestine is roughly 9 feet in length but gets it’s name because it has a larger diameter than the small intestine.
Your large intestine is also called the colon.
The large intestine is divided into several sections.
Transcript
http://www.moomoomathblog.com/....2021/01/all-about-la


Difference between Organic and Inorganic Compounds
Organic compounds contain carbon. There are at least four important organic compounds, lipids, proteins, carbohydrates, and nucleic acids.
Inorganic compounds do not contain carbon and usually are more simple compounds.
There are some compounds that contain carbon and are inorganic like carbon dioxide.
*
*
For more Life Science videos and summaries see,
http://www.moomoomath.com/Midd....le-School-Science-an


Introduction to metabolism | Biology|
You may have heard that you have a fast metabolism or a slow metabolism
But what exactly is your metabolism?
Metabolism is the sum of all chemical reactions within the cells of living things.
Take a look at this summary of all of the chemical reactions taking place in a human.
The chemical reactions of metabolism are organized into pathways in which one chemical is transformed through a series of steps into another chemical, with the help of enzymes.
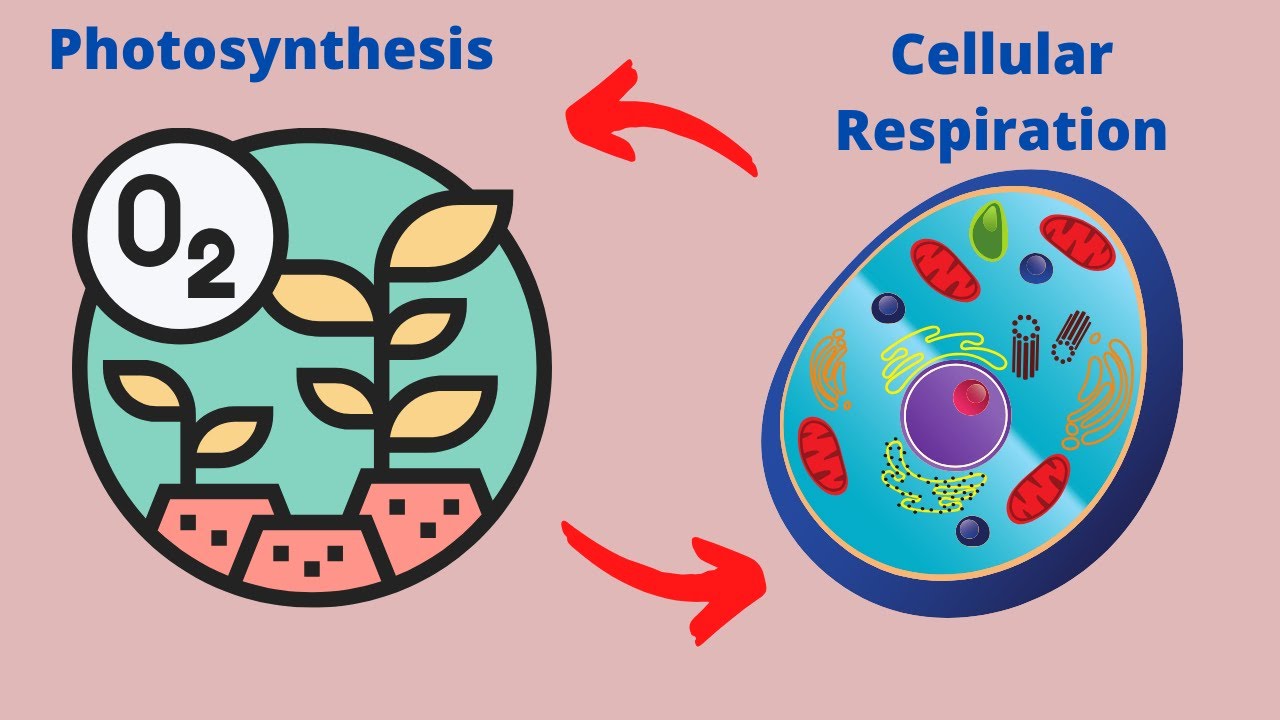
An example of a pathway is cellular respiration in which glucose and oxygen are transformed into carbon dioxide water and ATP which is used for energy
You can break metabolism down into three main categories.
The chemical reactions used for the conversion of food to energy
The conversion of food into building blocks used to make items like proteins
The chemical reactions needed for the elimination of waste
Enzymes are crucial to metabolism Enzymes help speed up chemical reactions The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products.
Almost all chemical reactions in the cell need enzymes in order to occur at rates fast enough to sustain life
There are over 5000 chemical reactions that occur in plants,animals, and microbes that are controlled by enzymes.
Most of the structures that make up animals, plants and microbes are made from three basic classes of molecule: amino acids, carbohydrates and lipids
Many chemical reactions focus on making these molecules during the construction of cells and tissues, or by breaking them down and using them as a source of energy during digestion and cellular respiration.
These chemicals can also be joined together to make DNA and proteins.
So there you go metabolism the sum of chemical reactions that take place in living things.
Take a journey to the cell
https://www.youtube.com/watch?v=QS274gwv_Sc
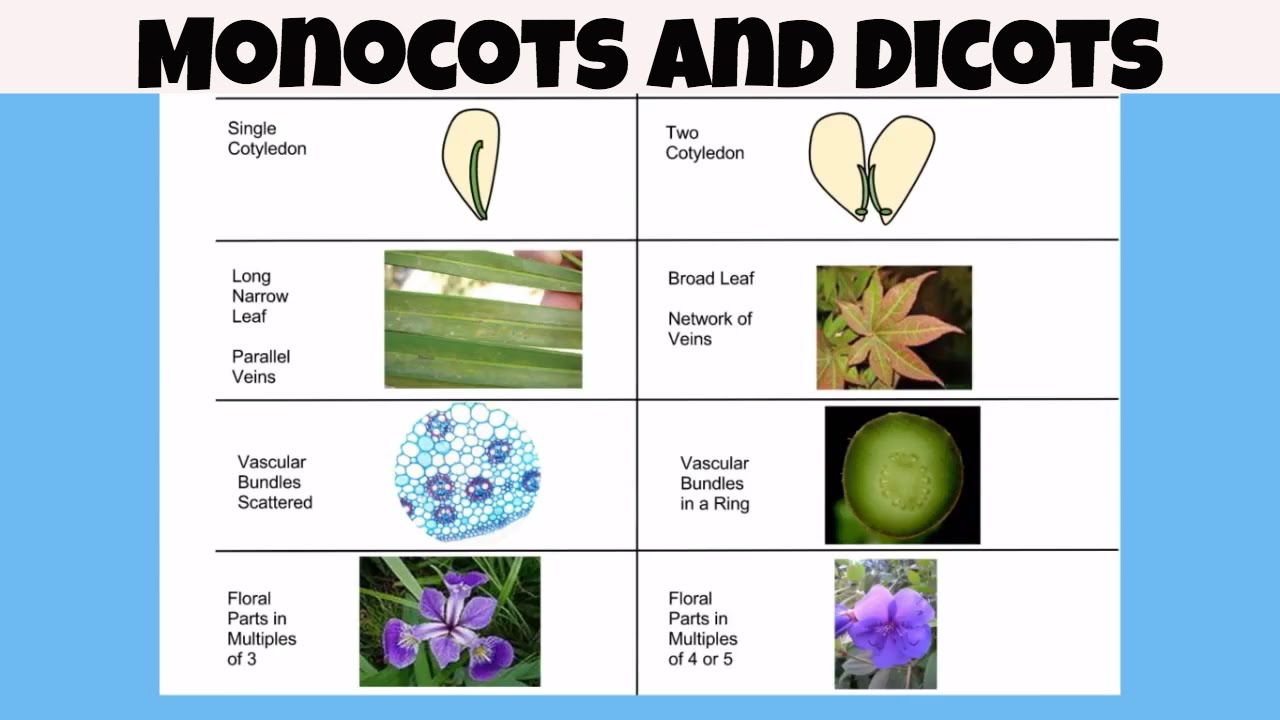

Difference between monocot vs dicot
Angiosperms are plants that have flowers and seeds encased in fruit.
Angiosperms can be divided into to major categories, monocots and dicots.
Monocots have one cotyledon and dicots have two cotyledons.
You may enjoy
Parts of a flower crossword puzzle
http://www.moomoomath.com/flow....er-parts-crossword-p
*
*
For more Life Science videos and summaries see,
http://www.moomoomath.com/Midd....le-School-Science-an


Learn the difference between prime and composite numbers. All whole numbers greater than one can be classified as prime or composite numbers.
Prime numbers have only factors one and itself. A composite number has other factors other than itself and one. So let's look at some examples. Let's look at 4 and 5
Four is divisible by 1 and four is divisible by 2 so four is a composite number
5 is divisible by 1 but it is odd so it is not divisible by 2 or any other number so 5 is prime.
More information
http://www.moomoomathblog.com/....2022/02/difference-p


Description of the major plate boundaries.
In the video, I include description of convergent boundaries. There are three types of convergent boundaries, continental to continental, oceanic to continental, and oceanic to oceanic.
Divergent boundaries are when lithospheric plates move away from each other.
Transform boundaries slide past one another.
How Tectonic Plates Move
http://www.moomoomathblog.com/....2021/03/how-tectonic
Plate Boundaries
http://www.moomoomathblog.com/....2020/02/6-plate-boun


In this video, I answer the question, " what makes an amphibian an amphibian?" Learn about the main characteristics of amphibians. Examples of amphibians include frogs,salamanders,newts,toads, and cecillians. All amphibians are vertebrates that can go through some type of metamorphasis.
More about Amphibians
http://www.moomoomathblog.com/....2022/04/amphibians.h


Many single-cell organisms, some plants, bacteria, and even animals have the ability to make copies of themselves.
In this video, I explain how fission, budding, spores, and regeneration allow unicellular and multicellular items to make copies of themselves.
Transcript
http://www.moomoomathblog.com/....2019/01/asexual-repr
https://moomoomath.com/asexual-reproduction/


Learn the positives and negatives of nuclear power.
In the ’70s people started getting excited about nuclear energy because it has the technology required to be used on a large scale.
In fact, nuclear energy accounts for roughly 21% of the electricity produced in the US.
Compare this to all of the other renewables like solar and wind which combined only produce 12%
But, nuclear power has a dark side
Nuclear Energy Transcript
http://www.moomoomathblog.com/....2021/12/nuclear-ener


Learn the difference between a pure substance and a mixture. A pure substance can be an element or a compound. A mixture can be homogeneous or heterogeneous.
In the video, I cover pure substances vs mixtures examples. Along with the differences between pure substances and mixtures.
More on Pure Substance and Mixture
http://www.moomoomathblog.com/....2022/06/types-of-sol
For more Math help visit our website
http://www.moomoomath.com/


The Kuiper belt is a collection of objects beyond Neptune. The asteroid belt is a region of asteroids found between Mars and Jupiter.
More on Asteroids-Meteroids-Comet
http://www.moomoomathblog.com/....2023/09/whats-differ
Kuiper belt object picture
https://www.flickr.com/photos/....tonynetone/369783481


Learn the difference between an endothermic reaction and an exothermic reaction.
Endothermic reactions take in energy and might be cold to the touch. Exothermic reactions give off energy and will sometimes be warm or hot to the touch.
At @MooMooMath and Science, we end our videos with kindness multiplies kindness. In each of my #shorts I like to tell a quick Kindness story in order to inspire myself and others to practice kindness.
Acts of kindness make a lasting positive impact on the people around you.
You can find all of my kindness stories in this playlist.
https://youtube.com/playlist?l....ist=PLurjkZV1ykGZs20
Chemical reactions
https://moomoomath.com/exother....mic-vs-endothermic-c