Top videos
Learn how to interpret words and phrases in the context in which they appear!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
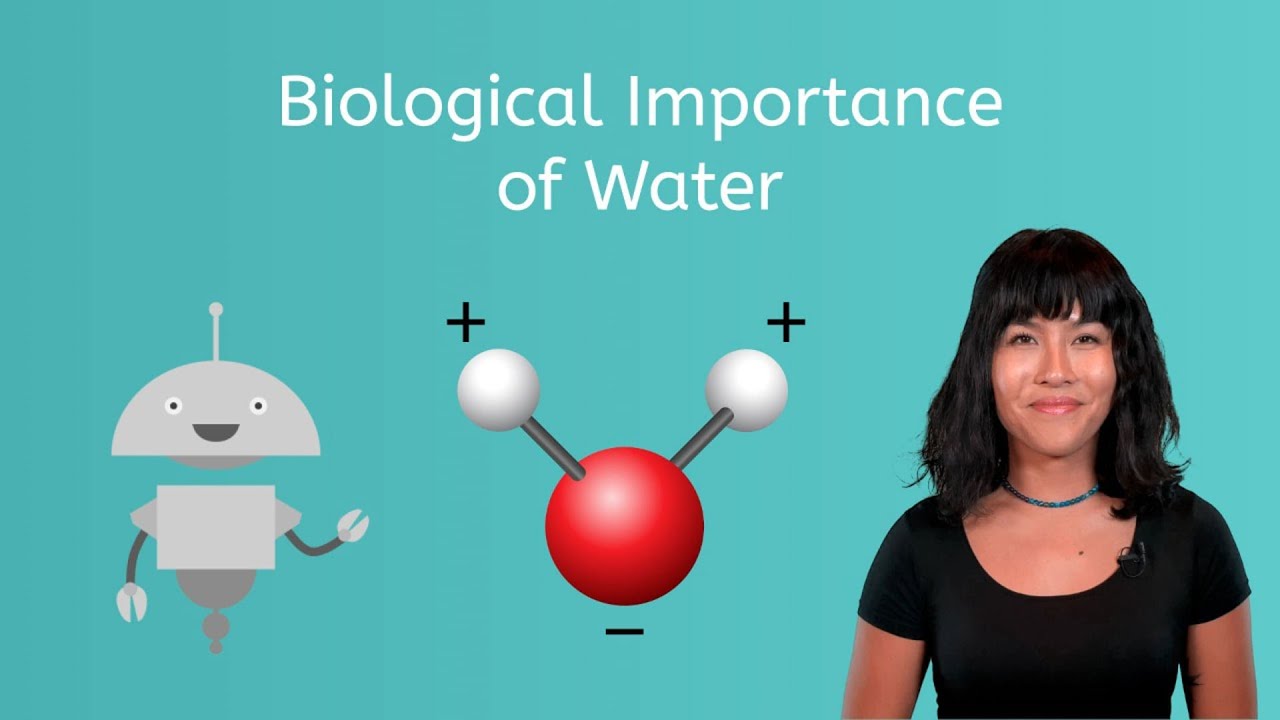
Learn about the biological importance of water.
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
In this video, you will learn how to read and recognize number words 31-40!
We hope you are enjoying this video! For more in-depth learning, check out Miacademy.co (https://www.parents.miacademy.co/), an online learning platform with lessons and practice games that go along with our videos. Use coupon code VIDEOSPECIAL for a discount. Good for homeschoolers, Parents, Teachers, or anyone with kids in grades K-8.
Miacademy also offers many hands-on learning activities including a business simulation, writing in the Miacademy weekly, and creating artwork. Teaching social skills is also one of the key values at Miacademy. On the site, kids can interact safely in a moderated environment and share their artwork, videos, and ideas with each other.
Learn all about our math puzzle challenge game.
We hope you are enjoying this video! For more in-depth learning, check out Miacademy.co (https://www.parents.miacademy.co/), an online learning platform with lessons and practice games that go along with our videos. Use coupon code VIDEOSPECIAL for a discount. Good for homeschoolers, Parents, Teachers, or anyone with kids in grades K-8.
Miacademy also offers many hands-on learning activities including a business simulation, writing in the Miacademy weekly, and creating artwork. Teaching social skills is also one of the key values at Miacademy. On the site, kids can interact safely in a moderated environment and share their artwork, videos, and ideas with each other.
Let's learn how to easily learn important phone numbers!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
In this video, you will look at a few different famous algebraic equations, and identify the parts!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Learn how to add more than two numbers together.
We hope you are enjoying this video! For more in-depth learning, check out Miacademy.co (https://www.parents.miacademy.co/), an online learning platform with lessons and practice games that go along with our videos. Use coupon code VIDEOSPECIAL for a discount. Good for homeschoolers, Parents, Teachers, or anyone with kids in grades K-8.
Miacademy also offers many hands-on learning activities including a business simulation, writing in the Miacademy weekly, and creating artwork. Teaching social skills is also one of the key values at Miacademy. On the site, kids can interact safely in a moderated environment and share their artwork, videos, and ideas with each other.
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
By the end of this lesson, you will be able to describe the factors that affect ecological stability, explain how ecological disturbances affect ecosystems, and differentiate between the two kinds of ecological succession.
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Get a 35% discount on our website - practice games, quizzes, and more to go along with each video: https://www.parents.miacademy.....co/coupon?code=VIDEO
What are the books of the New Testament? Find out in this video!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Learn how to make a crazy creature flip book, inspired by the creatures in the Amazon, part III.
We hope you are enjoying this video! For more in-depth learning, check out Miacademy.co (https://www.parents.miacademy.co/), an online learning platform with lessons and practice games that go along with our videos. Use coupon code VIDEOSPECIAL for a discount. Good for homeschoolers, Parents, Teachers, or anyone with kids in grades K-8.
Miacademy also offers many hands-on learning activities including a business simulation, writing in the Miacademy weekly, and creating artwork. Teaching social skills is also one of the key values at Miacademy. On the site, kids can interact safely in a moderated environment and share their artwork, videos, and ideas with each other.
Are you a food lover? Learn more about a career as a farm manager!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Learn about forms of financial exchange used in the past, the forms we use today, and what forms of financial exchange might be used in the future.
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you’d like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Learn about lines and angles.
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
In this video, learn how to put away dishes. We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Get a 35% discount on our website - practice games, quizzes, and more to go along with each video: https://www.parents.miacademy.....co/coupon?code=VIDEO
Learn about the physical characteristics of Oceania and Antarctica, economic activities in Oceania, and how Antarctica is governed!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you’d like us to cover any additional topics, please let us know.
For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
We hope you are enjoying our large selection of engaging core & elective K-12 learning videos. New videos are added all the time - make sure you come back often to learn more! If you'd like us to cover any additional topics, please let us know. For practice, assessment, and many interactive activities that go along with each video, as well as a teacher/parent dashboard, go to Miacademy.co for Grades K-8 or Miaprep.com for grades 9-12!
Get a 35% discount on our website - practice games, quizzes, and more to go along with each video: https://www.parents.miacademy.....co/coupon?code=VIDEO
In this video, you will learn all about the amazing animals of the ocean!
We hope you are enjoying this video! For more in-depth learning, check out Miacademy.co (https://www.parents.miacademy.co/), an online learning platform with lessons and practice games that go along with our videos. Use coupon code VIDEOSPECIAL for a discount. Good for homeschoolers, Parents, Teachers, or anyone with kids in grades K-8.
Miacademy also offers many hands-on learning activities including a business simulation, writing in the Miacademy weekly, and creating artwork. Teaching social skills is also one of the key values at Miacademy. On the site, kids can interact safely in a moderated environment and share their artwork, videos, and ideas with each other.